![]()
2022-05-20 updated
CYBERDYNE Inc. (Tsukuba, Ibaraki, Japan, President & CEO: Yoshiyuki Sankai, from now on referred to as the “Company”) announced today that an international journal on the HAL for Medical Use Lower Limb Type (hereafter “Medical HAL”) had been published by Toho University School of Medicine Department of Neurology in Internal Medicine*1 published. In this journal, the research group from Toho University School of Medicine reported on a one-year Cybernics Treatment™*2 using Medical HAL for patients with Amyotrophic Lateral Sclerosis (“ALS”). In this progressive neurodegenerative disease, improvement of muscle weakness cannot be expected with therapeutic drugs and other means, although some can inhibit the progression. All three patients showed an effect of maintaining their walking function with HAL Cybernics Treatment™. The average 2-minute walking distance remained at the same level as before the start of treatment at 300 days. Clinical trials have shown the efficacy of treatment with Medical HAL for eight types of progressive neuromuscular diseases, including ALS, which led to coverage of such treatment with public health insurance. However, this paper is an important achievement in the medical industry because it demonstrates the effectiveness of the treatment over a period of one year.
The research group that conducted this study included Assistant Professor Harumi Morioka (tenured), Lecturer Takehisa Hirayama, Professor Osamu Kano of the Department of Neurology, Toho University School of Medicine and Professor Satoru Ebihara of the Department of Rehabilitation Medicine, published a paper on March 17, 2022, regarding short-term (one to two months) Cybernics Treatment using a medical HAL for ALS patients. The Company notes that the difference with the journal posted on this occasion is that the relevant journal investigated the long-term effect (one year).
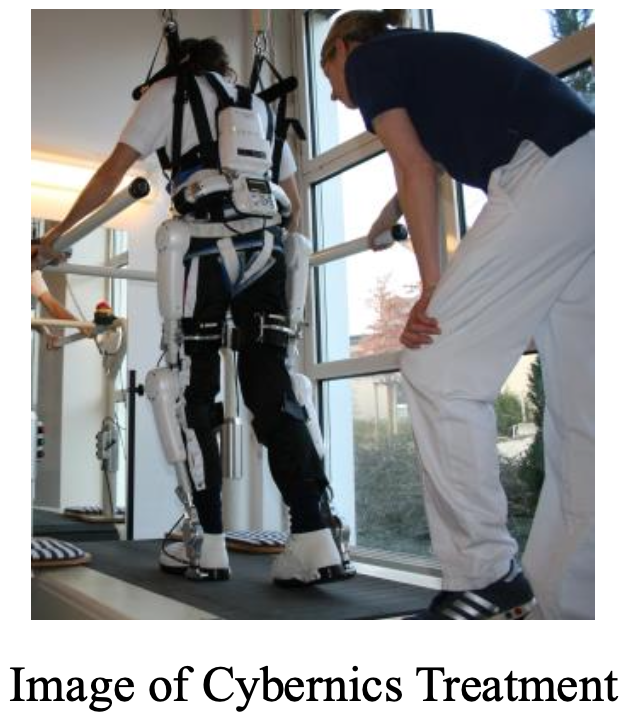
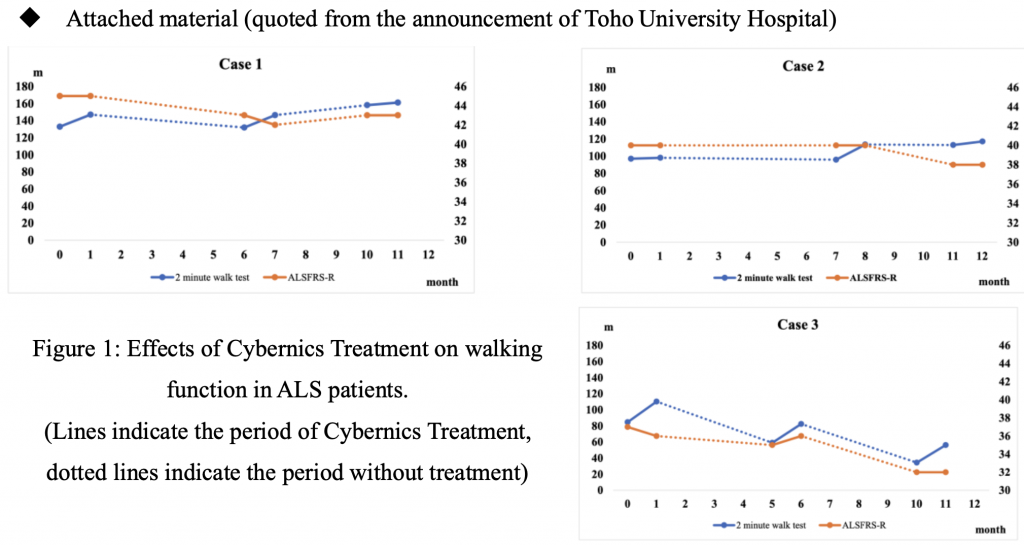
The study included three patients diagnosed with ALS at Toho University Hospital who received treatment between January and December 2019. All three patients recruited for the study could not walk independently and safely for more than 10 meters but could walk for more than 10 meters with assistance or walking aids. The evaluation method included three treatment courses of HAL (each course composed of nine sessions, frequency 2-3 sessions/week, for 1-2 months, duration: 20-40 minutes, excluding putting on HAL to the patient and breaks). 2-minute walking distance, 10-meter walking test (assessing speed, stride length, and walking rate), ALS motor function rating scale (ALSFRS-R), Barthel Index (BI), functional independence measure (FIM), and effort lung capacity were observed and analyzed before and after each treatment sessions. As a result, while significance was not observed due to a small number of subjects, the average walking distance after 3 phases increased by 16.61 m (p=0.21). Furthermore, cadence significantly increased by 1.3 steps (p=0.02) on average. The figure below shows that while the 2-minute walking distance was maintained by walking exercise using HAL, the ALSFRSR, a motor function evaluation scale for assessing motor functions such as swallowing function and upper limb ability of ALS patients, which were not covered by the HAL Lower Limb Type, decreased with the progression of ALS.
We want to take this opportunity to express our deepest gratitude to the entire staff of the Toho University ALS Clinic for their cooperation in producing the results of this study.
© 2025 CYBERDYNE INC. ALL RIGHTS RESERVED.