![]()
2021-09-02 updated
CYBERDYNE Inc. (Tsukuba, Ibaraki, Japan, President & CEO: Yoshiyuki Sankai) announced today that its application for a medical device marketing license for Medical HAL Single Joint Type, a Wearable Cyborg that improves physical functions, has been approved by the Australian Therapeutic Goods Administration, Department of Health (TGA). TGA registered the summary of the product on the Australian Register of Therapeutic Goods (ARTG)*1. The approved target diseases cover cerebrovascular disorders (stroke) and treatment after orthopedic surgery, as in the EU.
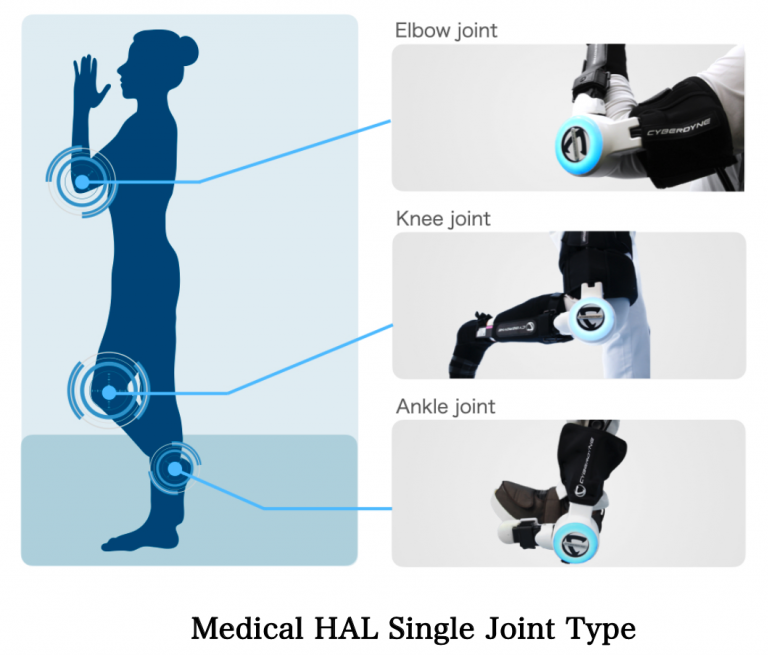
The Company has obtained medical device approvals in Japan, the U.S., and the EU for Medical HAL Single Joint Type. In the Asia-Pacific region (APAC), the approval of the medical device in Australia follows those in Malaysia and Thailand. In collaboration with our local partners, we will deploy Cybernics Treatment, an innovative medical technology originating from Japan, in Australia and contribute to realizing a society of health and longevity where all people can enjoy healthy and affluent lives.
*1After receiving evaluation for safety and functionality, a company must register their medical device sold in Australia in the Australian Register of Therapeutic Goods (ARTG) managed by the TGA.
Reference)
Public summary of Medical HAL Single Joint Type registered on ARTG
https://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStore.nsf&docid=46C122EF73343FD3CA25873D004245D3&agid=(PrintDetailsPublic)&actionid=1
August 30, 2021
CYBERDYNE listed Medical HAL Single Joint Type to U.S. FDA as Class I Medical Device
https://www.cyberdyne.jp/english/company/PressReleases_detail.html?id=11345
July 13, 2020
HAL Single Joint Type obtained certification as a medical device for Japan, applied for insurance coverage
https://www.cyberdyne.jp/wp_uploads/2020/07/20200713_PR_eng.pdf
October 8, 2019
HAL Single Joint Type obtained conformity certification as a medical device for EU
https://www.cyberdyne.jp/wp_uploads/2019/10/20191008_PR_Single-Joint_ENG.pdf
© 2025 CYBERDYNE INC. ALL RIGHTS RESERVED.