![]()
2022-02-09 updated
At today’s Japanese Central Social Insurance Medical Council Meeting, the council approved the reported revision plan for the insurance reimbursement pricing from April 1, 2022. The revision plan included revision for the treatment of Medical HAL Lower Limb Type.
■ Outline of the revision plan
1)Added to the excluded items from Diagnosis Procedure Combination*

*J118-4 Gait Treatment (by robot suit) that were previously calculated based on Diagnosis Procedure Combination (DPC) will be calculated each time the treatment is conducted. This will enable hospitals adopting the DPC system to calculate insurance for treatment with HAL towards hospitalized patients.
Reference document 3 “Method of Calculating the Amount of Expenses Required for Medical Treatment in Wards of Hospitals Designated by the Minister of Health, Labour and Welfare” page 2
(available in Japanese only)
https://www.mhlw.go.jp/content/12404000/000894877.pdf
2) Increase of insurance reimbursement pricing
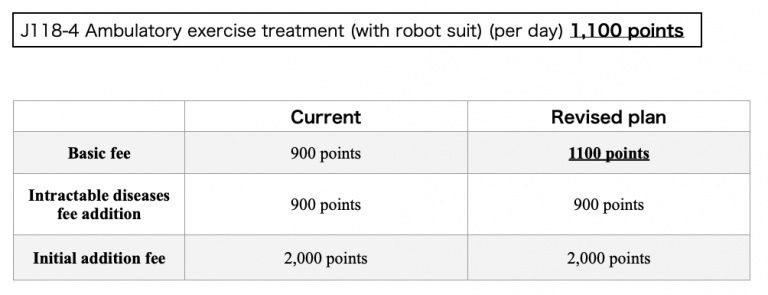
** Basic fee related to J118-4 Gait treatment (by robot suit) will be increased from 900 to 1100 points.
Reference document 1-1 “medical fee” page 200 (available in Japanese only)
https://www.mhlw.go.jp/content/12404000/000894873.pdf
*Spinal muscular atrophy, spinal and bulbar muscular atrophy, amyotrophic lateral sclerosis, Charcot-Marie-Tooth disease, distal muscular dystrophy, inclusion body myositis, congenital myopathy, muscular dystrophy
Excerpts from the Proposal to Evaluate the Medical Technology (why it should be reevaluated) submitted by the Japanese Society of Neurological Therapeutics – Translated by CYBERDYNE
“The medical technology produced significant improvement of physical function towards slowly progressive neuromuscular disease, which are intractable diseases with no established treatment methods that are effective. The medical effect observed was unheard of by any existing treatment methods, including pharmaceuticals approved for these diseases. Due to the progressive nature of these intractable diseases, research on the natural course of the disease suggest a gradual decline of motor functions. However, when this medical technology was utilized repeatedly over a long duration of 3.5 years, an opposite trend was suggested, and motor function was maintained/improved. Furthermore, the medical technology did not increase the destruction of the patient’s muscles. The CK value in the blood** was actually in the declining trend, which is medically noteworthy. Thus, it was suggested that medical technology is a safe treatment method for progressive neuromuscular patients. The medical technology should no longer be regarded as a treatment method to support gait exercises. It should be reevaluated as a new treatment method to activate the loop of the patient’s brain-nerve systems.”
**Note by CYBERDYNE. Creatine Kinase in the blood is an assessment index to show the disability in the musculoskeletal system. Conventional exercise therapy progresses the destruction of the muscles and increases the CK value.
© 2025 CYBERDYNE INC. ALL RIGHTS RESERVED.