![]()
2022-02-07 updated
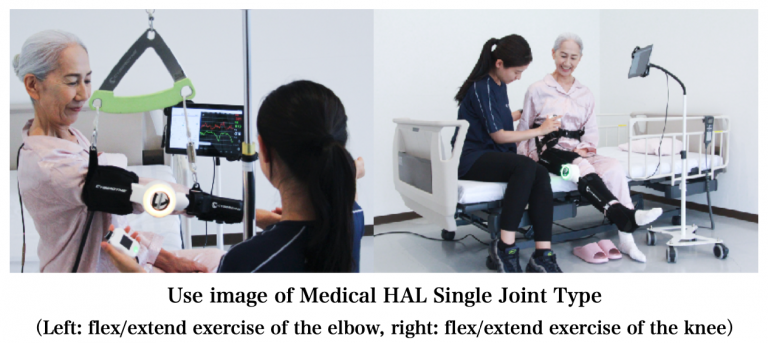
CYBERDYNE Inc. (Tsukuba, Ibaraki, Japan, President & CEO: Yoshiyuki Sankai) announced today that its application for a medical device marketing license for Medical HAL Single Joint Type, Wearable Cyborg™ that improves physical functions, has been approved by the Ministry of Health Republic of Indonesia (Kementerian Kesehatan Republik Indonesia, from now on referred to as the “Ministry of Health”) and that the summary of the product has been registered on its database. The approved target diseases cover cerebrovascular disorders (stroke) and treatment after orthopedic surgery, as in the EU.
Video of Medical HAL Single Joint Type used for Cybernics Treatment
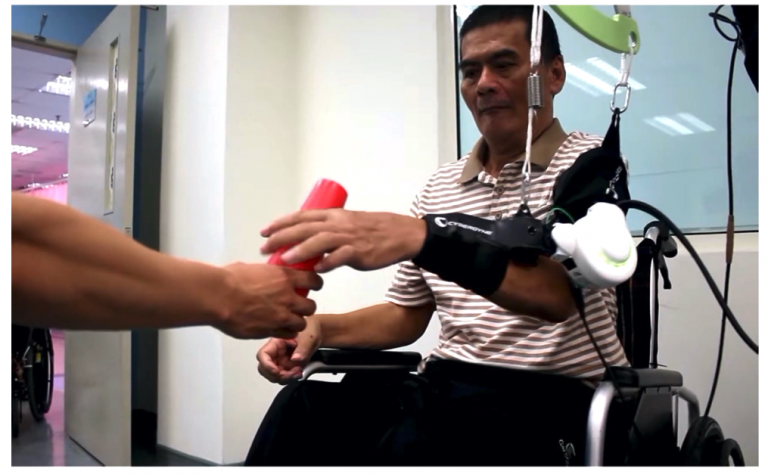
(Credit: SOCSO Tun Razak Rehabilitation Centre) :https://youtu.be/01NuuGUscIE?t=22
Reference) the summary of the product registered by the Ministry of Health
https://cyberdyne.jp/wp_uploads/2022/02/Cyberdyne-SIngle-Joint-MDA-AKL.pdf
Product page of Medical HAL Single Joint Type
https://www.cyberdyne.jp/english/products/SingleJoint_medical.html
© 2025 CYBERDYNE INC. ALL RIGHTS RESERVED.