![]()
2024-12-13 updated
CYBERDYNE Inc. (the “Company”) obtained conformity certification to the EU Medical Device Regulation* for its Small model of HAL for Medical Use Lower Limb Type on December 12, 2024.
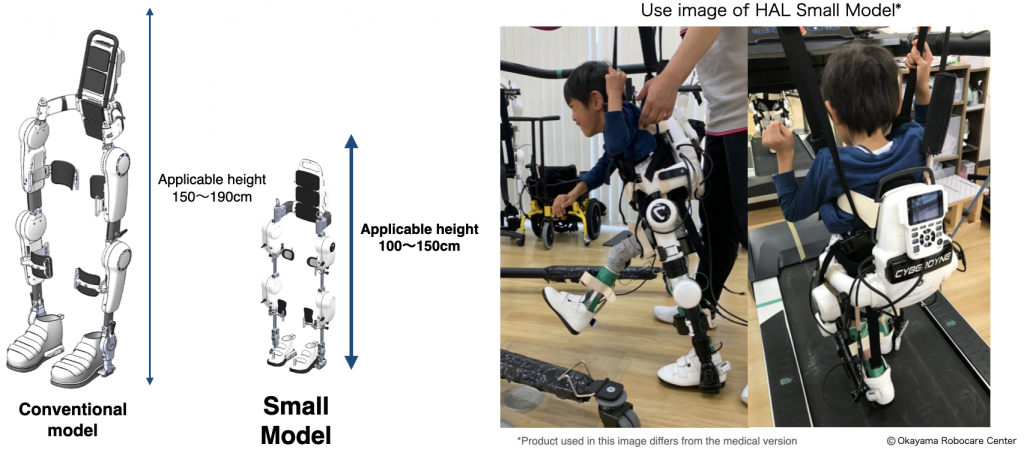
Before this conformity certification, only patients eligible for Cybernics Treatment were those above 150 cm. EU patients from 100 cm to 150 cm (approx.) can go through Cybernics Treatment.
The Company received approval for this small model in the USA in May 2024 from the U.S. Food & Drug Administration. This conformity certification will enable the Company to advance its effort to obtain approvals for this model in other regions.
*Third-party certifier verified that the device conforms to the new EU Medical Device Regulation (MDR) that became effective in 2021. The device can now be labeled with a CE Mark and be sold in the EU.
© 2026 CYBERDYNE INC. ALL RIGHTS RESERVED.