![]()
2025-01-31 updated
CYBERDYNE Inc. (Ibaraki, Tsukuba, Japan, President & CEO: Yoshiyuki Sankai; from now on, referred to as the “Company”) obtained certification to manufacture and sell its small-size model of Medical HAL (Official trade name: Medical HAL Lower Limb Type B; from now on, referred to as “the Device”) in Japan, on January 31, 2025, from the Japanese Minister of Health, Labour and Welfare.
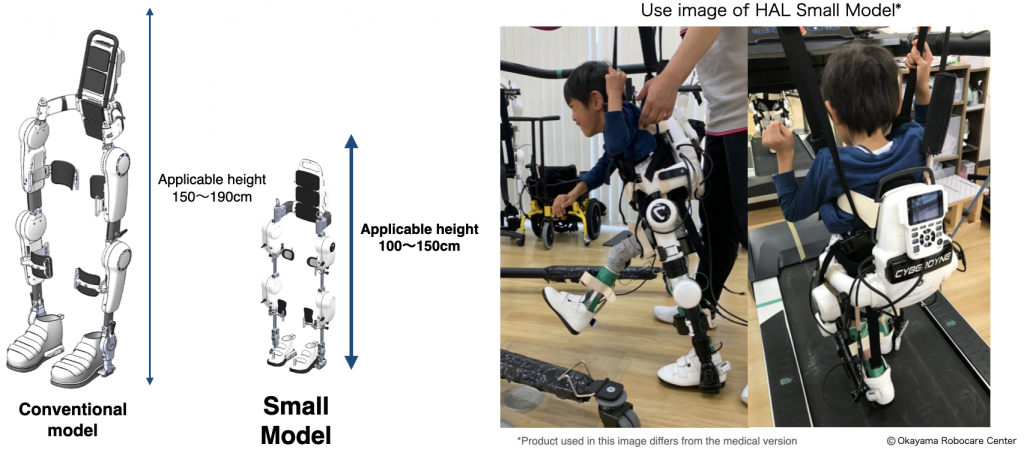
The application for the Device was submitted to the Japanese Pharmaceuticals and Medical Devices Agency in June 2023 as a medical device for the approved target diseases*1. The approval will enable patients from 100 cm to 150cm to access Cybernics Treatment, as they could not undergo treatment due to the minimum size requirement.
Following this event, the small-size model is now approved in the U.S., Europe, and Japan, the three major markets for medical devices. The Company will now apply for insurance coverage in Japan for this model. The Company will continue its efforts to make the Device available worldwide and further accelerate its medical HAL business of CYBERDYNE Group.
About CYBERDYNE
CYBERDYNE Inc. is a future pioneering company that simultaneously creates innovative technologies, creates new industries, and fosters human resources through these challenges by utilizing Cybernics (*) that fuse “Human” and “Cyber Physical space” (HCPS) to solve various issues facing society. Through these challenges, the Company promotes a virtuous cycle of innovation. The world’s first wearable cyborg, HAL, a representative product of Cybernics technology, has been recognized for its high efficacy and safety in promoting functional regeneration as part of Cybernics treatment. Its expansion has progressed to over 20 countries and regions worldwide. CYBERDYNE Group aims to create a safe and secure society where individuals of all generations can enhance their independence and freedom, addressing various challenges related to daily life and physical and mental well-being. CYBERDYNE focuses on supporting those with health, physical function, cognitive, or psychological difficulties, as well as people working across diverse sectors of society.
Reference
*1 Target diseases
Spinal Muscular Atrophy (SMA), Spinal and Bulbar Muscular Atrophy (SBMA), Amyotrophic Lateral Sclerosis (ALS), Charcot-Marie-Tooth disease (CMT), Distal Myopathy, Inclusion Body Myositis (IBM), Congenital Myopathy, Muscular Dystrophy, HTLV-1 associated myelopathy (HAM), Hereditary Spastic Paraplegia
*2 (June 21, 2023) Application for medical device approval for the addition of Medical HAL small model
https://www.cyberdyne.jp/english/company/PressReleases_detail.html?id=13462
*3 (May 15, 2024) U.S. FDA becomes the world’s first to clear smaller model of Medical HAL and expanded indication for Cerebral Palsy
https://www.cyberdyne.jp/english/company/PressReleases_detail.html?id=14025
*4 (December 13, 2024) Small model of HAL for Medical Use obtained conformity certification as a medical device for the EU
https://www.cyberdyne.jp/english/company/PressReleases_detail.html?id=14589
© 2026 CYBERDYNE INC. ALL RIGHTS RESERVED.